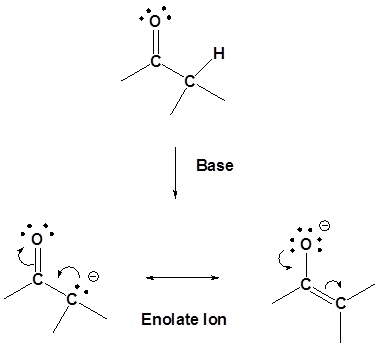

Alkyl hydrogen atoms bonded to a carbon atom at an α (alpha) position relative to a carbonyl group display unusual acidity. While the pKa values for alkyl C-H bonds is typically on the order of 40-50, pKa values for these alpha hydrogens is more on the order of 19-20. This can most easily be explained by resonance stabilization of the product carbanion, as shown:
The enolate has two resonance forms – the negative charge can be either on carbon or oxygen – but enolates usually react as nucleophiles from the carbon, as we saw in section 9.7. with enolate alkylation SN2 reactions.
When we first studied enolate chemistry previously in section 9.7, we used LDA to generate enolates which then reacted as nucleophiles in SN2 reactions. For these alkylation reactions to be useful, the enolate anions must be generated in high concentration in the absence of other strong nucleophiles and bases. Aqueous base (e.g., aq. NaOH) and alkoxides (e.g., NaOCH2CH3) are usually not be suitable because they produce only low concentrations of the enolate anions, and the remaining -OH or -OR can cause unwanted side reactions. In other words, these nucleophilic bases will simply react directly with the alkyl halide via an SN2 reaction.
Some bases that have been successfully used for enolate anion formation are: NaH (sodium hydride, pKa > 45), NaNH2 (sodium amide, pKa = 34), and LiN[CH(CH3)2]2 (lithium diisopropylamide, LDA, pKa 36). Ether solvents like tetrahydrofuran (THF) are commonly used for enolate anion formation.  Because of its solubility in THF, LDA is a widely used base for enolate anion formation. In this application, one equivalent of diisopropylamine is produced along with the lithium enolate, but this normally does not interfere with the enolate reactions and is easily removed from the products by washing with aqueous acid. Many ketones form enolates cleanly with LDA, for example cyclohexanone:
Because of its solubility in THF, LDA is a widely used base for enolate anion formation. In this application, one equivalent of diisopropylamine is produced along with the lithium enolate, but this normally does not interfere with the enolate reactions and is easily removed from the products by washing with aqueous acid. Many ketones form enolates cleanly with LDA, for example cyclohexanone:  Although the reaction of carbonyl compounds with sodium hydride is slow, sodium enolates are formed with the loss of hydrogen, and no other organic compounds are produced.
Although the reaction of carbonyl compounds with sodium hydride is slow, sodium enolates are formed with the loss of hydrogen, and no other organic compounds are produced.

 Formation of enolates from esters and nitriles" width="528" height="204" />
Formation of enolates from esters and nitriles" width="528" height="204" />
Unfortunately, aldehydes do not react cleanly with strong bases to form enolates, so enolate formation with LDA is normally only performed with ketones. However, some other related structures such as esters and nitriles can also form stabilized carbanions with LDA similar to ketone enolates, and these can react in similar ways to ketone enolates:
If the formed enolate is stabilized by more than one carbonyl it is possible to use a weaker base such as sodium ethoxide to form the enolate almost quantitatively.

Enolates are very useful in synthesis, as they represent a stabilized nucleophilic form of carbon. This chart shows the range of reactions that can be used:

We will examine the aldol reaction next. The Claisen condensation will be covered later, in section 22.2.
We previously saw (section 9.7.) how enolates act as nucleophiles in SN2 reactions. But they can also act as nucleophiles to attack carbonyls via a nucleophilic addition reaction (similar to what we saw earlier in this chapter with Grignard reagents and LiAlH4). When enolates are used for additions in this way, the reaction is a useful carbon-carbon bond-forming reaction known as the Aldol Reaction. Here, an aldehyde (or ketone) forms its enolate, which then reacts with a second molecule of aldehyde (or ketone) to form a beta-hydroxy aldehyde (or ketone) by alpha C–H addition of one reactant molecule to the carbonyl group of a second reactant molecule. For this reaction to occur at least one of the reactants must have α hydrogens.
If a base is added a low temperatures to an aldehyde or ketone that has an alpha hydrogen, an enolate is formed which immediately undergoes a nucleophilic addition across the C=O of another molecule of aldehyde or ketone. As long as the reaction is kept cold, a beta-hydroxyaldehyde (often called an “aldol”) or a beta-hydroxyketone product can be isolated. Since the only electrophile present is the aldehyde/ketone, a weaker base such as NaOH or NaOCH3 can be used.

If the reaction is warmed, it can then lose a molecule of water to form an alkene-aldehyde or alkene-ketone, known as an alpha,beta-unsaturated aldehyde or ketone. In this case the overall reaction is known as an aldol condensation.
An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone.

Aldol condensations are important in organic synthesis, because they provide a good way to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or “aldol” (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals.

The name aldol condensation is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, the aldol reaction is not formally a condensation reaction because it does not involve the loss of a small molecule.
The reaction between an aldehyde/ketone and an aromatic carbonyl compound lacking an alpha-hydrogen (cross aldol condensation) is called the Claisen-Schmidt condensation. This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. G. Schmidt, who independently published on this topic in 1880 and 1881. An example is the synthesis of dibenzylideneacetone. Quantitative yields in Claisen-Schmidt reactions have been reported in the absence of solvent using sodium hydroxide as the base and plus benzaldehydes.
The first part of this reaction is an aldol reaction, the second part a dehydration—an elimination reaction (Involves removal of a water molecule or an alcohol molecule). Dehydration may be accompanied by decarboxylation when an activated carboxyl group is present. The aldol addition product can be dehydrated via two mechanisms; a strong base like potassium t-butoxide, potassium hydroxide or sodium hydride in an enolate mechanism, or in an acid-catalyzed enol mechanism. We will focus on the base-catalyzed mechanism, which is more widely used.
If the catalyst is a moderate base such as hydroxide ion or an alkoxide, the aldol reaction occurs via nucleophilic attack by the resonance-stabilized enolate on the carbonyl group of another molecule. The product is the alkoxide salt of the aldol product. The aldol itself is then formed, and it may then undergo dehydration to give the unsaturated carbonyl compound. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself.
Base-catalyzed aldol reaction (shown using − OCH3 as base)
Base-catalyzed dehydration
Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a stoichiometric amount of a strong base such as LDA. In this case, enolate formation is irreversible, and the aldol product is not formed until the alkoxide of the aldol product is protonated in a separate acid-base workup step. Mixtures of stereoisomers (E & Z) are obtained from some reactions, though the E product is generally favored. Overall the general reaction involves a dehydration of an aldol product to form an alkene:
Going from reactants to products simply

Figure: The aldol condensation example